Building FDA-Compliant Medical Electronics: 10 Engineering Decisions That Define Your Regulatory Success
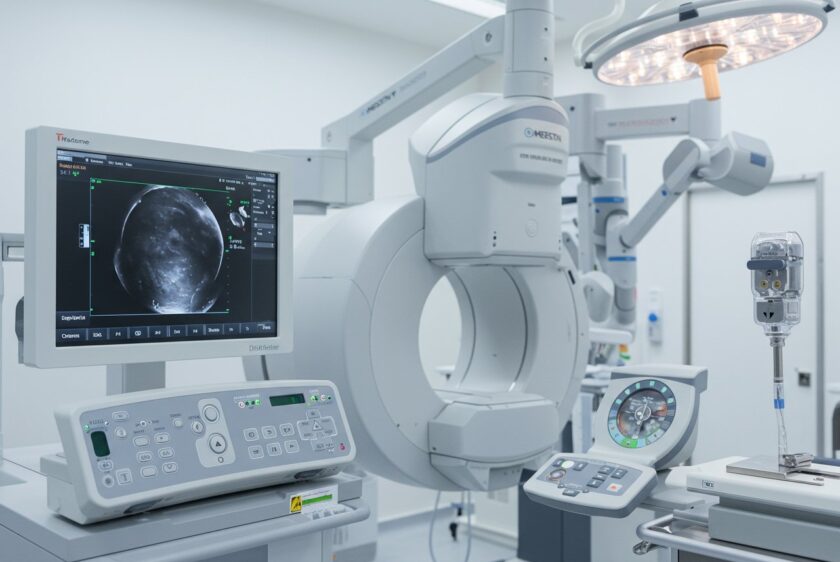
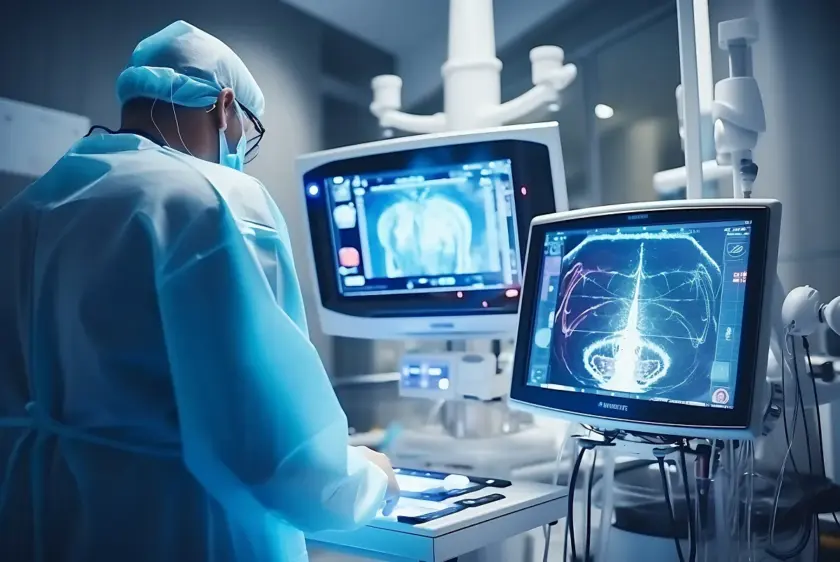
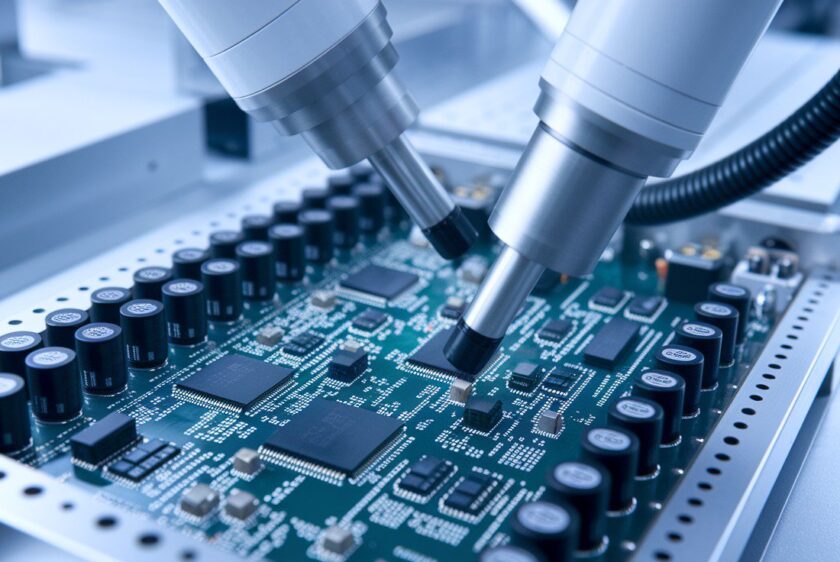
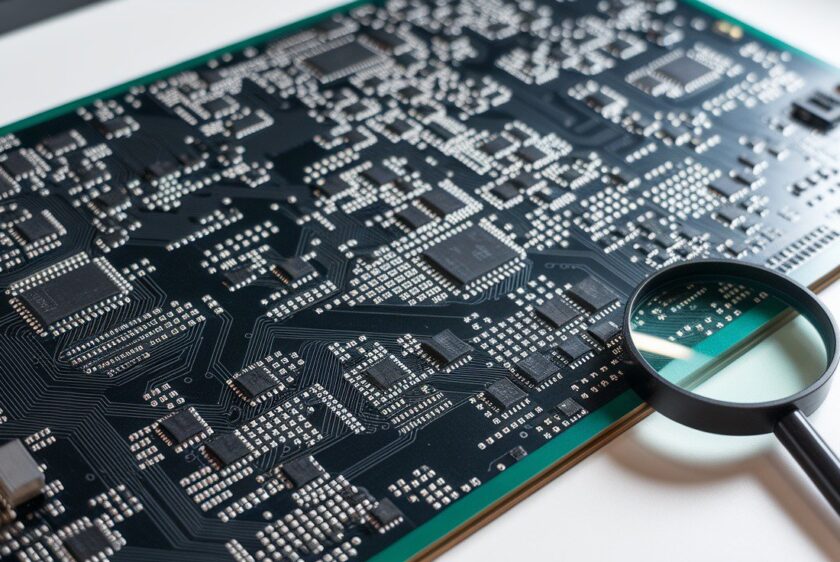
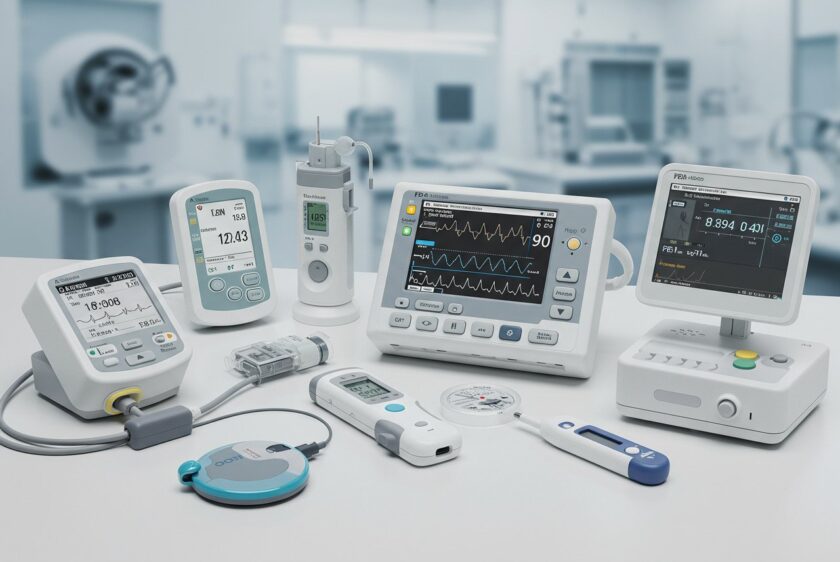
Building medical devices goes beyond technical specs and aesthetic prototypes—it's about stringent compliance and validation processes. From initial design classification to robust documentation and risk management, it’s essential to embed compliance into your engineering workflow. Discover how to navigate FDA regulations, ensure traceability, and integrate risk management seamlessly without compromising on innovation or efficiency.